
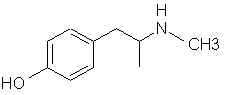
Now considering this reaction would only involve 4 reactions, 2 of which are ester related (high yielding, almost quantitative and uses cheap reagents). Then if you deesterfied the phenylalanine and decarboxylated you should be left with D-Amphetamine. Now I am not entirely familiar with this field of chem so correct me if I'm wrong, but to my understanding if you esterified L-phenylalanine then did an enolate alkylation you would be left with L-alpha methyl phenylalanine ethyl ester. This is where enolate chemistry comes in. Theoretically if you could alkylate phenylalanine at the alpha position and then decarboxylate you would be left with D-amphetamine. Unfortunately for us most phenylalanine is L isomer, and would reduce into L-amphetamine, not to mention the expensive reducing reagents. One of the best remaining precursors is phenylalanine, mostly because it is unregulatable. A single step synthesis is obviously not available anymore, and even most 2 step synthesis have been regulated. So as governments crack more and more down on amphetamine synthesis we have been forced to move further and further away from direct synthesis.


 0 kommentar(er)
0 kommentar(er)
